I. Introduction
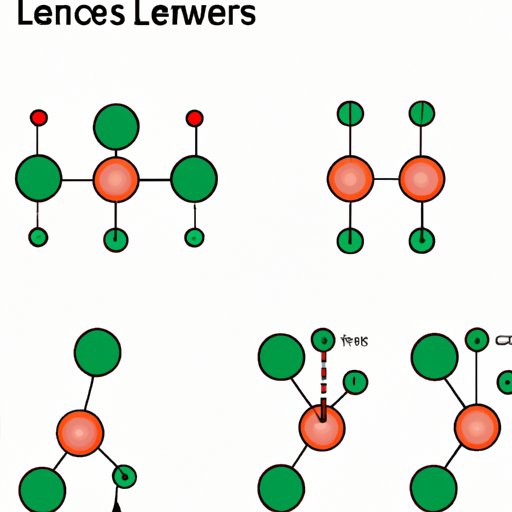
When it comes to understanding chemical reactions, it’s important to have a good grasp of molecular structures. The Lewis structure, named after Gilbert Lewis, is a way of displaying the arrangement of atoms and their electrons in a molecule. Being able to draw Lewis structures accurately is crucial in predicting reactions, determining the geometry of molecules, and understanding chemical bonding. In this article, we will guide you through the step-by-step process of drawing Lewis structures, highlight common mistakes to avoid, explore real-world applications, and give you practice problems to test your understanding.
II. Step-by-Step Guide
Step 1: Count the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Valence electrons are the electrons in an atom’s outermost shell.
Step 2: Determine the central atom in the molecule. In most cases, this will be the atom with the lowest electronegativity or the atom that can form the most bonds. Hydrogen and halogens are never central atoms.
Step 3: Use single bonds to connect the central atom with each of the other atoms in the molecule.
Step 4: Distribute the remaining valence electrons around the atoms in the molecule, giving each non-central atom an octet of electrons and the central atom an octet or duet.
Step 5: If the central atom does not have an octet, try double or triple bonds, creating resonance structures if necessary.
Example: Drawing the Lewis structure for methane (CH4)
Step 1: Carbon has four valence electrons, and each hydrogen has one valence electron. Thus, we have 4 + 4 = 8 valence electrons in total.
Step 2: Carbon is the central atom in this molecule, and it can form four bonds.
Step 3: We connect each hydrogen to carbon using a single bond.
Step 4: We distribute the remaining four valence electrons around each hydrogen, giving each atom an octet.
Step 5: Carbon already has eight valence electrons, so the molecule is complete.
Drawing Lewis structures can be more complicated for molecules with multiple central atoms or with atoms with more than eight valence electrons, but the basic steps are the same. Practice drawing Lewis structures for various molecules to get a feel for the process and improve your accuracy.
III. Common Mistakes
One of the most common mistakes when drawing Lewis structures is not counting the valence electrons correctly. Make sure to double-check your electron count before moving forward in the process. Additionally, some atoms can form multiple types of bonds (single, double, or triple), which can be confusing. Remember that the goal is to give each atom an octet or duet of electrons, and sometimes multiple types of bonds are necessary to achieve this.
Another common mistake is incorrect placement of lone pairs of electrons. These should always be placed on outer atoms before the central atom, and should be placed in the same plane as the atoms. Avoid placing lone pairs on the central atom when possible, as it can result in unneeded formal charges.
Accuracy is essential when drawing Lewis structures, as subtle changes can have a tremendous impact on the predictions of chemistry. Make sure to avoid careless mistakes and double-check your work before moving on.
IV. Visual Aids
Visual aids, such as colorful diagrams and animations, can help learners better understand the process of drawing Lewis structures. These aids can demonstrate how electrons move around the molecule, how resonance structures form, and how the final structure should look. Make sure to choose visual aids that are accurate and effectively demonstrate the concepts. Online resources can be an excellent source for interactive visual aids that can give you a hands-on approach to learning Lewis structures.
V. Real-World Applications
Lewis structures are incredibly important in chemistry, as they can predict the reactivity and physical properties of molecules. Chemists use Lewis structures to determine bond polarity, molecule polarity, acidity, and basicity of a molecule. By understanding the Lewis structure of a molecule, chemists can make predictions about the molecular geometry, boiling points, melting points, and many other features that are necessary to understand chemical reactions.
In addition, Lewis structures are used in the design of new drugs, fertilisers, and many other chemical compounds needed for our everyday lives. Without a good understanding of Lewis structures, it can be impossible to create new molecules with the required properties.
VI. Practice Problems
To test your understanding of Lewis structures, practice problems can be a valuable tool. Make sure to choose problems that range in difficulty to test different aspects of understanding. Practice problems can be found in many textbooks, online resources, and even worksheets from professors.
For example:
Determine the Lewis structure for sulfur hexafluoride (SF6). How many shared pairs and unshared pairs of electrons are on the central atom?
By practicing a variety of Lewis structures, you can increase your proficiency and accuracy, and be better prepared for real-world problems.
VII. FAQs
Q: How do I draw Lewis structures for ions?
A: Ions follow the same basic rules as neutral molecules, but with an adjusted number of electrons. For cations, subtract electrons from the total electron count, and for anions, add electrons.
Q: What is a resonance structure?
A: A resonance structure is a structure that can be drawn for a molecule or ion in which one or more atoms have multiple possible locations for electrons. These structures can be interspersed to represent the actual electron distribution in the molecule.
Q: How many lone pairs can an atom have?
A: The maximum number of lone pairs an atom can have depends on the atom’s position on the periodic table. Most atoms can only accommodate two or three lone pairs, while others are limited to zero or one. Make sure to double-check your sources for any exceptions to this rule.
VIII. Conclusion
Drawing Lewis structures is a necessary skill for anyone interested in understanding chemical reactions. By following the basic steps, utilizing visual aids, practicing problems, and avoiding common mistakes, you can become proficient in drawing and interpreting these molecular structures. Remember that practice makes perfect, and keep honing your drawing skills to become a master of Lewis structures.