Introduction
Chloroform is a chemical compound that has been widely used for centuries. It was once commonly used in anesthesia and as a cleaning agent, but its use has diminished due to the health risks associated with exposure. In this article, we will explore the composition and properties of chloroform, its uses, risks, and safety precautions, as well as its history and availability. We will also break down its chemical structure and molecular behavior and discuss potential alternatives for certain applications.
Exploring the Composition and Properties of Chloroform: What You Need to Know
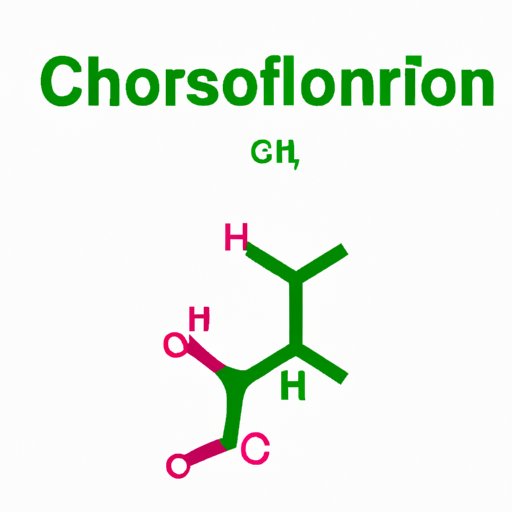
Chloroform has the chemical formula CHCl3, meaning it is composed of one carbon, one hydrogen, and three chlorine atoms. It is a colorless liquid with a sweet smell and taste and can be synthesized by reacting acetone with bleaching powder. Chloroform is soluble in alcohol, ether, and benzene, and it has low solubility in water.
Chloroform has a high refractive index, meaning that it bends light more than most other liquids. It also has a relatively high boiling point of 61.15 °C and a density of 1.488 g/cm³. Additionally, chloroform is highly reactive with other organic compounds and can undergo a range of chemical reactions.
A Closer Look at Chloroform: Its Uses, Risks, and Safety Precautions
Chloroform has had many uses over the years, particularly in the medical field. It was once used as an anesthetic but has since been replaced by safer alternatives, such as ether and nitrous oxide. Currently, chloroform is primarily used in industry as a solvent for fats, oils, and resins.
Exposure to chloroform has been linked to a number of health risks, including liver damage and respiratory problems. It is also a known carcinogen, meaning it has the potential to cause cancer. Therefore, safety precautions are essential when handling chloroform. These include wearing protective clothing and gloves and working in a well-ventilated area with adequate ventilation and air filtration. It is also important to avoid ingestion or direct skin contact with chloroform, as this can cause severe health problems.
Understanding Chloroform: Its History, Availability, and Medical Applications
Chloroform was first discovered in 1831 by American physician Samuel Guthrie. He found that it could be used as an anesthetic during surgery, and it was widely used throughout the 19th century until its dangers became known. Today, chloroform is no longer used in the medical field except in rare cases where no other anesthesia is available, such as in developing countries with limited medical resources.
Although chloroform is now primarily used as an industrial solvent, it is still available for purchase in some areas. However, due to its potential health risks, caution should be exercised when using or storing it.
Exposing the Dangers of Chloroform: Risks and Precautions for Safe Handling
As mentioned, exposure to chloroform can cause a range of health problems, including liver damage, respiratory problems, and even cancer. Therefore, it is important to take precautions when handling chloroform. These include wearing protective clothing and gloves, using adequate ventilation and air filtration, and working in a well-ventilated area.
It is also important to avoid direct skin contact or ingestion of chloroform. If exposure occurs, it is recommended to seek medical attention as soon as possible. To prevent accidental exposure, chloroform should be stored in a clearly labeled container in a secure location away from children and animals.
Chloroform: Breaking Down Its Chemical Structure and Molecular Behavior
Chloroform’s chemical structure and molecular behavior play an important role in its various properties and uses. The molecule is composed of one carbon atom, one hydrogen atom, and three chlorine atoms, arranged in a tetrahedral shape. Chloroform is a polar molecule, meaning it has a positive and negative end, which makes it highly reactive with other organic compounds.
Chloroform is commonly used as a solvent due to its ability to dissolve fats, oils, and resins. It is also used in the production of refrigerants and some pesticides. Chloroform’s properties make it useful in these applications, as it can undergo a range of chemical reactions.
From Anesthesia to Cleaning Products: The Versatile Uses of Chloroform and Its Implications
Chloroform has had diverse uses over the years, from its initial use as an anesthetic to its current use as an industrial solvent. However, its health risks have led to a shift away from its use in most applications. Many industries have turned to safer alternatives, and some countries have even banned its use altogether.
It is important to recognize the implications of the continued use of chloroform in certain applications. While it may be effective in some cases, the potential health risks associated with its use make it an unsuitable option for many industries. As such, alternatives should be explored wherever possible.
Conclusion
Chloroform is a chemical compound with a range of properties and uses, but it also poses significant health risks. Its initial use as an anesthetic paved the way for further medical advancements, but its dangers have led to its replacement with safer alternatives. Today, chloroform is primarily used as an industrial solvent and should be handled with caution due to its potential health risks. Understanding the chemical structure and molecular behavior of chloroform can provide insight into its various properties and applications. Ultimately, it is essential to prioritize safety and explore alternatives wherever possible.